InteractionsGuide Index Page
Analysis Search Terms:

Preface
We are in the midst of a critical historic juncture in the evolution of medicine. Three major currents are influencing the practice of medicine, the science underlying its methodology, and the relationship between caregiver and patient. First, a significant proportion of patients are exercising greater levels of self-education and self-care, requesting greater opportunity for informed decision making and often demanding a realignment of the usual power relationships in conventional medicine. Second, the emergence of personalized medicine, particularly but not only in the form of genomics, pharmacogenomics, and nutrigenomics, offers the promise of tailoring efficacy while decreasing adverse effects in a manner that offers a modern scientific approach to individualization of care beyond generic pathology and lowest-common-denominator generalizations. Third, the now well-established recognition of medical pluralism mandates greater communication and collaboration between health care providers of various medical traditions from diverse cultures and philosophies, methodologies and therapeutics. Together, these factors give a profoundly transdisciplinary impetus to the development of enhanced clinical strategies synthesizing the science of medicine and the art of healing in a process-oriented approach to the individual patient.
Within the context of these converging influences, the issues arising from the potential interactions between conventional drug therapies and herbal and nutritional therapies present both a challenge and an opportunity. The challenge involves unanticipated adverse drug reactions (ADRs). The opportunity involves the potential for enhancing the depth and breadth of the mainstream medical model, ultimately improving the success of clinical outcomes. After more than two decades of complementary and “alternative” medicine (“CAM”) and subsequent optimistic portents of an era of “integrative medicine,” the average practitioner surveying the medical landscape for substantive changes in the clinical care model usually sees only glimmers of vision, moments of inspiration, and select ideal cases. Such aspirations may be suitable for some, but most clinicians would be content simply with effective tools for understanding other disciplines, how their patients are using them, and the implications when mixing pharmaceutical medicines and other modalities. Nevertheless, our patients are using a diverse array of medical approaches, and we all are entering a world of transdisciplinary care and personalized medicine, complexity models of physiology, and unprecedented access to information. In this context, the discovery of new synergies and unparalleled collegial collaboration offer the promise of enhanced patient empowerment and novel clinical strategies. Ultimately, we must always keep in mind that our first and only loyalty can be to our patients and their health, even if it forces us to grapple with the unfamiliar and acknowledge the unconventional.
Purpose and Function
The present work derives from the clinical needs of practicing health care professionals who have been compiling, analyzing, and publishing assessments on interactions for two decades. Interactions between drugs and nutrients first became a subject of review and education in 1988 during the development of the Integrative BodyMind Information System (IBIS) database. On publication of that reference tool, clinicians increasingly requested deeper coverage of interactions issues involving nutrients and herbs, in response to widespread and growing use of such products by their patients and clinicians’ desire to integrate such agents into their therapeutic repertoire. In 1997, exhortations from physicians and educators precipitated a dedicated focus on interactions issues in a clinical context and catalyzed development of the Interactions software database, subsequently published in 2000. That reference work essentially functioned as the first edition of the research and writing that has evolved into the present publication. Through that experience and several years of input and feedback from health care professionals and educators around the world, as well as inquiries from an online reporting and resource website, the models, methods, and tools applied in the creation of this publication were developed and refined. In particular, a year of intensive investigation, dialogue, and debate produced the pioneering system of interaction characterization, literature evaluation, and clinical probability applied in reviewing, compiling, and assessing the relevant scientific and medical literature. Thus, the current volume represents the indirect outcome of almost 20 years of work and 10 years of direct efforts.
Interactions between pharmaceutical drugs and “natural” products such as nutrients and herbs constitute an area of immediate concern and growing awareness wherever patients are receiving health care and members of the public are engaged in self-care through use of these substances. Many patients naively assume that nutrient and herbal products are “natural” and that this somehow implies they are “safe.” More disturbing is the acknowledged fact that many patients often withhold disclosure of nutrient and herbal intake or use of nonconventional care from their conventional health care providers. This combination suggests the need for deep inquiry and analysis into the doctor-patient relationship and the coordination of medical care. In the meantime, however, clinicians need a guide to working with patients using nutrients and herbs and guidelines for crafting their interventions to coordinate these elements. This book is most directly intended to serve this need among health care professionals, educators, and students throughout the whole range of medical professions. The depth of coverage, the emphasis on research, and the focus on practical implications address the needs of this professional audience. Secondarily, this information will be of benefit to individuals in other professions, ranging from librarians to retail staff, faced with questions from the general public regarding these issues.
An overview of the presently available literature reveals the predominance of two types of print and electronic publications covering this subject matter: those intended for the professional market and those intended for a consumer/patient audience. The same data may also be disseminated differently for use by both audiences. In both cases, most publications can fairly be characterized as lacking in depth and incomplete or overreaching in conclusions.
The professional literature tends to assume that patients using nutrients and herbs (“dietary supplements”) are doing so without supervision of health care professionals trained and experienced in the therapeutic application of nutritional and botanical therapies. Lack of disclosure by patients also tends to be the case, and greater awareness, frank discussion, and informed decision making in these areas are necessary for effective clinical management as well as patients’ perception of respect for their choices. Similarly, especially in complex and chronic conditions, the use of multiple providers from several different health care disciplines is increasingly common. Professionals in all disciplines can increase clinical efficacy and patient safety through open dialogue and collegial (peer-to-peer) collaboration. However, interactions literature is often prepared by health care professionals without training or experience in nutritional and botanical therapies. Therefore, the advantages of purposefully combining, strategically sequencing, or deliberately avoiding such interventions are usually not addressed in a comprehensive and practical way. In addition, the interactions reference texts and guides developed for consumers are limited in depth, often avoid critical issues, and sometimes prey on readers’ fears by exaggeration and headline hyperbole. Furthermore, the commercial context of their use frequently presents an apparent conflict of interest, especially when provided as aids in product selection in a retail setting. Similarly, these guides are presented as online educational tools appended to broader coverage of “alternative medicine” within the context of medical, pharmaceutical, or insurance websites. Either situation implies significant constraints as to thoroughness, rigor, and efficacy because caution is justifiably the operative premise. The issue of apparent conflict of interest and possible bias must always be considered in a setting funded and presented by commercial interests, both for what is said and what is avoided. Inherently, the scope, thoroughness, and standards of evidence tend to be different for consumer education (or promotional) publications than for professional publications because of the vast divergence in educational level, operational needs, and responsibility. Overall, the literature available to most of the general public, whether magazines, books, friends and family, or the Internet, tends to avoid recommendation of these agents at therapeutic doses or in a manner that might be construed as treating diagnosed conditions.
By contrast, in this book we explore many methods of using nutritional and herbal interventions not only as “dietary supplements” but also as components of comprehensive, transdisciplinary therapeutic strategies. This approach diverges fundamentally from typical drug interactions databases marketed for physicians, especially those for handheld devices, that list telegraphic warnings (often unsubstantiated) about “supplements” without context or qualification. Where concomitant administration of nutrients and herbs might harm a patient or significantly interfere with predictable effects of conventional medications, the priority of ensuring patient safety is established throughout the text by providing cautions and suggesting modifications based on scientific literature and clinical experience.
Methodology and Structure
The methodology applied in developing this publication involved two steps. First, input was gathered from among the authors and other clinicians, as well as pharmacists and educators, as to potential interactions they were seeing in clinical practice and of substantive clinical relevance, particularly potential risk or observed therapeutic value. Second, available reference works on interactions, including drug-drug interactions literature, were reviewed for scope, depth, methodology, and presentation. This method ensures that we incorporated the best of all approaches, building on those from our own experience, and avoiding or countering those methods and characteristics that lead to poorly founded, overreaching, or misleading conclusions. Ultimately, the team that produced this book was determined to make realistic assessment of clinical relevance and evidence quality its foundation. Only a product that would meet the standards of what we would feel confident using in our own practices would be acceptable for publication. Thus, the research and editorial team consists of practicing health care professionals trained in therapeutic nutrition, botanical medicine, pharmacology, and pharmacognosy with experience on a daily basis in general family practice and specialty care, with an emphasis on internal medicine, oncology, and hematology. This text is therefore itself a result of collaboration among practicing experts from different modalities that is emblematic of the transdisciplinary collaboration that ultimately is required to understand and manage drug-nutrient and drug-herb interactions. This fact alone distinguishes this text from other publications that claim to cover the topics but present one-sided, theoretical, or partial approaches that necessarily lack the transdisciplinary insight and clinical relevance of this collaboration. Perhaps most importantly, our authors are deeply enmeshed in collaborative care on a daily basis, bringing together health care professionals from multiple disciplines and combining therapies from multiple modalities.
In areas where the available resources appeared to be limited, immature, or flawed, the authors systematically addressed three primary needs. First, the lack of a multidisciplinary approach inherent in the existing literature leads to the common tendency, for example, to assert potential interactions imputed from assumed pharmacological theory, without any corroboration from clinical experience with the interacting agents. Other than research into certain well-known drug-induced nutrient depletion patterns, direct clinical trials of adequate power are nearly absent from literature pertaining to drug-herb and drug-nutrient interactions. Overall, the primary source evidence tends to be delayed and reactive, fragmented, or tangential to other research objectives, or simply unapparent to those not trained and experienced in these fields. Consequently, many interactions supported by readily available evidence are simply not described in most texts. Also, many interactions based on substandard or preliminary evidence are given more weight than they deserve. In such cases, contextualization and “debunking” of the putative or alleged interactions often result from the necessary “reality check.” This problem also derives from overreliance on secondary literature (e.g., reviews, meta-analyses) and derivative material in topical publications. Second, no previously available text, including our first effort, incorporates sufficient detail to assess effectively the quality of the original data; the strengths and limitations of the study design, size, and duration; or the characteristics or even the dose, form, or other critical particulars of the agents involved. Third, clinical relevance and therapeutic implications need to be the primary goal of analysis of all interactions.
In light of these many factors, this text relies primarily on evidence from clinical trials whenever possible, using reasonable extrapolations from human research as secondary evidence and relegating animal studies or in vitro experiments to a supportive role. Published case reports are assessed based on the strength of their relevance, detail, and quality. Extrapolations can be reasonable or not. Our in-depth examination of the published literature revealed that the available secondary and derivative literature contains at worst an abundance of confusion and at best a lack of clarity on decisive facts, such as proper identification of a nutrient or herb and the plant part used, naturally occurring nutrients or whole herbs versus synthetic forms or extracts, doses outside the range of those considered safe and clinically effective, diverse modes of administration, and distinctive or even divergent characteristics of subject populations. In particular, the repeated citation of a poor-quality case report, irrelevant finding, or flawed research in multiple publications suggests superficial recycling of citations by secondary authors without full text review of original sources or critical analysis of conclusions. The pervasive interchanging as equivalents of Ephedra (the plant) and ephedrine (a pharmaceutical alkaloid) provides a stark illustration of this problem and its reverberations, despite the presumed good intentions of those making the assertions. In contrast, this text notes if studies use forms of an agent that experienced practitioners would shun, or doses too high for safety or too low for therapeutic efficacy. Perhaps most important is the recognition that uninformed administration of agents, or combinations of agents, in a manner wholly detached from the logic, methodology, or standards and guidelines of appropriate clinical practice and historical context from which they are derived, does not constitute an “adverse event” or “interaction.”
A significant gap often exists between the research literature and clinical practice in regard to the origins and form of many agents studied in the scientific literature. Not all nutrients or botanically derived preparations can accurately be described as “natural” medicines; many do not appear in nature and are more accurately placed in the province of “pharmaceutical preparations” rather than herbs or nutrients as traditionally understood or historically practiced. Thus, a gradient can be articulated spanning pharmaceutical nutrients (e.g., dl-alpha-tocopherol, i.e., racemic synthetic vitamin E), isolated preparations of naturally occurring nutrient and botanical constituents, foods and herbs in whole or minimally modified forms (with inherent diversity and variability of constituents), and nutrients and herbs in combinations or formulae (with even greater complexity). Often the isolated constituents and synthetic preparations or mixed isomers used in research studies contrast sharply with the forms (whether naturally occurring or crafted in accordance with traditional methodologies) typically used by practitioners trained and experienced in nutritional and botanical therapeutics. Moreover, the issues affecting interactions analysis are confounded by the recurrent paradox observed in innumerable papers on broader topics in which authors voice contradictory assumptions and conclusions; first, that whole-food sources are safer and more effective than, and generally preferable to, nutrients (or herbal) constituents in pill or tablet form, but second, that foods, herbs, nonstandardized extracts or isolates, or multicomponent formulae do not fit the methodology of study designs oriented to assessing single-agent pharmaceutical interventions. The resulting statements that naturally occurring and synthetic forms are equivalent, and recurrent findings that food (or herbal) sources provide benefits not found in synthetic supplemental or extracted forms, at least not in those specific forms studied, suggest that naturally occurring forms, identical in origin, to food (or plant) sources may be the missing link. Further, authors of research papers, and even more so those who read abstracts or press releases and then write articles on these papers for the professional and lay press, often fail to discriminate between studies using naturally occurring forms, dosage levels, and modes of administration typical in experienced clinical practice and studies using isolates or synthetic variants. Consequently, ill-founded extrapolations and unsubstantiated conclusions and warnings often result that diverge from the actual research findings and even more so from real-world clinical implications. In general, it might be stated that foods are a common preference among practitioners emphasizing nutritional therapies in their clinical practice. However, the therapeutic efficacy of food sources can be limited by several critical factors, including difficulty in achieving necessary dose thresholds of specific nutrients or constituents on a consistent basis because of volume of food sources required, low rates of compliance in the regular consumption of such required doses of necessary food items, and, in more recent decades, declining nutrient quality of commercially available foods.
Similarly, just as there is a large divergence between primary literature and the secondary literature of reviews and meta-analyses, an even greater separation exists between primary sources and editorial news coverage in both professional publications and popular press, as well as in educational materials and tools available to the typical patient/consumer. In particular, three primary limitations appear on analysis. First, the nature of study populations is often not defined, particularly with reference to critical distinctions between healthy subjects and those with diagnosed conditions. The related factors, such as genetic risk and pharmacogenomic variability, gender and age, health status, and socioeconomic vulnerabilities, are only considered on rare occasions. Second, critical factors in study design, such as cohort size, dose, form, duration of administration, and other potentially confounding influences, can significantly alter outcomes and the interpretation of findings. In many cases, these variables would be significant enough to create major divergences in assessing conclusions because characteristics of a given study could plausibly alter effects, but such characteristics were not mentioned or factored into the analysis and conclusions. Third, certain health care professionals, most notably naturopathic physicians and herbalists of the Euro-American, Chinese–East Asian, and Ayurvedic traditions, employ botanical formulations and nutritional therapeutics as a primary clinical intervention. Whether in these settings or in multidisciplinary integrative approaches, the use of multiple agents within a therapeutic strategy is typical practice, and especially in herbal medicine, single agents are rarely expected to provide the properties, potency, or personalization that a compound formula provides. Such treatment strategies are very difficult to assess adequately using research models or methodologies that assume single-agent interventions, which are uncommon in patients with chronic diseases and complex cases.
The methodology applied in this text emphasizes that supplementation of nutrients (and rarely, nutritive use of herbs) in healthy populations is distinct from unsupervised, concomitant use of nutrient support in individuals concurrently taking prescription drugs (especially those with known nutrient-depleting effects) and from purposeful coadministration of nutrients or herbs with conventional drugs within the context of clinical management by health care professionals applying integrative principles. Therefore, the organization of these interactions monographs involves a spectrum of categories, including “avoidance,” “benefit,” and “management.”
By incorporating summary tables as well as in-depth analysis of each interaction analysis, our text provides a useful combination of brevity and thoroughness by presenting an accurate overview as well as answering needs for deeper access to substance and detail. We refer to this publication as a “comprehensive” reference work because our goal is to articulate the subject in a broad and practical manner, not merely to catalogue available data without reference to origins, therapeutic context, patterns of usage, and clinical implications. In response to feedback from users of the previous software publications and in pursuit of strategic design goals, the current text not only increases the breadth and depth of the topics under consideration, but more importantly also applies a systematic method of analysis and presentation aimed at enhancing the clinical utility of the reviewed information and subsequent analysis. The structure and presentation of each monograph are designed to enable rapid review through summary analysis and coded characterizations of the character, significance, and evidence quality of each substantiated interaction, while also providing greater depth with a thorough review of evidence, mechanisms, and evolution of the scientific literature on the subject.
Each monograph provides basic information on physiology and function of the given nutrient or herbal agent, followed by a summary of established interactions and a review of clinically relevant data that contextualizes the discussion of interactions with specific drugs, including known or potential therapeutic uses and historical/ethnomedicine precedent, deficiency symptoms, dietary sources, nutrient preparations, typical therapeutic and supplemental dosing, laboratory assessment, and safety profile, with nutrient adverse effects, contraindications, and precautions and warnings. The Strategic Considerations section of each monograph discusses the broader clinical role of the particular agent, emphasizing implications of interactions, and further assesses the clinical relevance of the available data, patterns of interactions, and contentious or unresolved issues; this section also presents broad clinical reviews for substances for which substantive interactions evidence is lacking. Each review of a particular interaction dyad is divided into those with substantive evidence, primarily focusing on clinical trials and qualified case reports, and those relying on preliminary, speculative, and/or questionable data. Within each interaction pair, the pharmaceutical agent, and/or drug class, is introduced by generic name(s) and a summary characterization regarding type(s) of interaction(s) involved, quality of the evidence base, and estimated probability of clinical relevance, followed by subsections presenting mechanism(s) of action, evidence and practical clinical implications, suggestions, and cautions.
Presenting the development of evidence chronologically and thematically places emphasis on critical factors such as study design, number and characteristics of subjects, study duration, form, and dosage. Data from in vitro experiments and animal research are primarily used to examine possible mechanisms of action and to elucidate or qualify evidence from human research. In general, the standards applied in evaluating the strength of evidence are less demanding than those appropriate to evaluation of clinical trial relevance. Furthermore, patient safety is emphasized as the highest value, with a focus on optimizing outcomes while minimizing adverse effects. Overall, given the emerging state of interactions literature, the threshold of inclusion applied in weighing the available evidence is often of a lower standard than ideal, and conclusions are often qualified as such, with the oft-repeated recommendation that “further research through well-designed and adequately powered clinical trials is warranted.” Specific citations are appended to each monograph, and a separate file listing Reference Literature underpinning the overview presentations for each nutrient monograph is available.
The default bias often displayed in the medical literature assumes that interactions involving herbs and nutrients result in adverse effects. Not only are such outcomes merely one of several possible effects, but such reports are also often of substandard quality in the professional literature and are typically caricatured and misrepresented in the popular press. In assessing the quality of evidence, we have appreciated the taxonomy articulated by Fugh-Berman and Ernst (2001) for the review and assessment of report reliability in the area of herb-drug interactions. As noted in their meta-analysis, the data used in making claims of interactions are frequently inadequate and unreliable. Thus, these authors concluded that more than two thirds of published reports reviewed were “unevaluable” and graded only 13% as “well-documented.” They also noted that, in contrast to most drugs that contain a single pharmacological agent, most herbal products in use, and thus likely to be involved in possible interactions, tend to contain a variety of pharmacologically active constituents. The typical use of multiple herbs within a formulation further complicates the possibilities of ascribing causal relationships.
The literature of nutrient-drug interactions is typically not much better in quality than that of herb-drug interactions, with the possible exception of research into drug-induced nutrient depletions. Generally, the research cited is limited by several factors: inadequate patient history; presence of concurrent conditions or pathologies; involvement of one or more medications (particularly those with known interactions or adverse effects); lack of adequate recording of such comedications; failure to adequately describe, assay, or otherwise document alleged interactors; incomplete chronology or consideration of time relation of intake among substances; and incomplete consideration of alternative explanations for adverse effects. The need for improvements in the methodology, gathering, and analysis of reports and research of interactions involving herbs and nutrients will benefit all concerned and enable distinctions among harmful, beneficial, and bimodal interactions and clarify the frequency, intensity, and risk parameters influencing such events. Our conscious intention in assessing data is to tilt toward safety and emphasize conservative, nontoxic interventions. The development of this text has also emphasized avoidance of unreasonable and poorly founded extrapolations, especially speculative construction of “backwards interactions” based on tenuous assumptions. For example, asserting that because an herb may relieve symptoms assumed to derive from estrogen deficiency, the herb inherently will adversely interact with agents intended to elevate estrogen levels; or when a nutrient or herbal preparation demonstrates a beneficial effect on glycemic control, it is somehow interpreted as adversely “interacting” with hypoglycemic medications. In fact, however, such effects often represent a therapeutic synergy worth investigating.
Awareness of narrow therapeutic index, titration in response to gradual introduction
or withdrawal of any agent, and individualized assessment and evolving interventions
stand as the recurrent issues and key operative watchwords in safe and effective
implementation of interactions in a clinical setting. Overall, developing methods
for approaching potential interactions can help health care professionals avoid
inherently dangerous situations; minimize, neutralize, or counter risks and
potential adverse effects or impaired therapeutic responses; and engineer increased
therapeutic potency through additive, synergistic, or strategic combinations.
Many of the limitations in the scientific literature relating to interactions
reflect and parallel those in the broader study of herbal and nutritional therapies
in general. The scientific literature available to those attempting to assess
the probability and clinical significance of interactions among various agents
is inherently incomplete and inclined to be reactive. Whether in the area of
drug-drug interactions or those involving nutrients or herbs, the instances
of purposeful research into adverse interactions are rare. Case reports and
circumstantial findings tend to dominate the literature and are of highly variable
quality, with the clear majority qualifying as incomplete and unreliable. Further,
although the scientific study of combined drug and nutrient therapies is emerging,
and study of drug-induced nutrient depletions is occasionally commanding attention
(again, usually in reaction to accumulating reports of adverse outcomes), the
literature investigating drug-herb interactions is in a highly preliminary,
and often woeful, state. Again, unqualified case reports tend to dominate the
headlines, and only recently have experienced practitioners of herbal medicine
become involved in designing clinical trials or evaluating case reports. Notably,
most discussions of herbs in the conventional literature involve single herbs,
and if being used for treatment, then only using a generic, pathology-focused
treatment model, when in fact experienced health care professionals almost
always prescribe herbs in formulae that are modified to match peculiar individual
patient characteristics and that evolve over time in response to changes in
the patient's condition. Similarly, the dosages, clinical indications, prescribing
methodology, preparation methods, and even plant parts used in most published
trials often reflect little on the professional practice of herbal medicine.
In other words, the two groups are usually talking about different things,
and the designs of most studies have limited relevance to clinical practice
of European, American, or East Asian schools of herbal medicine. Thus, we see
a surge in discussion of the use of St. John's wort for depression, when a
poll of professional herbal prescribers before such papers would have revealed
no consistent use of the plant for that condition as a broad psychiatric diagnostic
category; perhaps for melancholy and head injuries, but not “depression.” Even
more disturbing are studies using parts of plants never used or rarely used
by herbalists or in doses at grossly different levels of potency than typical
in clinical practice. Similar issues arise when looking at studies of vitamins,
minerals, or other nutrients where single-agent interventions using forms usually
avoided by professional prescribers, and often at doses considered ineffectual,
produce insignificant outcomes. “Well, what would you expect; that's
why we don’t do it that way,” is the usual response from experienced
botanical/nutritional practitioners. The major consequences of such ill-conceived
research are wasted money and resources and lost opportunities at evolving
scientific knowledge and collegial collaboration.
The secondary literature discussing interactions involving herbs and nutrients amplifies and distorts the problems with the primary source material. Again, “news” tends to be hyperbolic and inflammatory and information delayed, reactive, and overrun with incestuous overuse of poorly qualified and superficially analyzed or incomplete reviews of the primary literature; consequently the conclusions are often poorly founded and hasty, of questionable clinical relevance, and misleading in their therapeutic implications. “The devil is in the details,” and more often than not, no one bothered to look into the details. Although not nearly as “glamorous” and headline producing as adverse events and dangerous interactions, the areas of drug-induced nutrient depletions and integrative interventions combining drugs, nutrients, and/or herbs represent the bulk of substantive interactions material. Beyond simply correcting problematic interactions resulting from ignorance and lack of communication, these potential avenues of purposeful interactions management also constitute the most promising opportunities for the development of scientific knowledge capable of delivering the clinical interventions most likely to result in successful outcomes. Again, we see the necessity of distinguishing between supplemental use of nutrients, especially vitamins and minerals, on a broad level for undifferentiated populations and the clinical application of botanical and nutritional agents as therapeutic interventions in their own right or as part of multicomponent strategies. The quantity and quality of clinical trials focusing on, or at least taking note of, interactions will grow as researchers in conventional medicine become more conversant with nutrient and herbal therapies and engage practitioners experienced in such modalities in study design. All these observations emphasize the need to develop tools to facilitate submission of clinical data with complete and pertinent details to high-quality case reports of herb-drug-nutrient interactions, with the active cooperation of all parties involved. High-quality case reports can form the basis for meaningful clinical research and allow the emergence of informed and clinically relevant pharmacovigilance.
Grappling with drug-drug interactions is well known for its difficulties, and the emerging field of drug-nutrient and drug-herb interactions introduces many other complications, as mentioned. Because interactions involving drugs are commonplace and can be dangerous, those involving nutrients and herbs need not seem particularly unusual. Most notoriously, as one noted PharmD commented in conversation: “We just assume that warfarin interacts with everything.” Drug-drug interactions are known for variability based on dose, timing, gender, hepatic function, and other drug clearance parameters. Reliably predicting interactions involving more than two agents, of any type, inherently invokes greater uncertainty, and any declarations other than probability are best viewed with skepticism. As a result, throughout the interactions literature, including that covering drug-drug interactions, there is often an implicit understanding that such interactions are strictly pharmacodynamic or pharmacokinetic and thus completely within the province of pharmacology. On close examination, however, such assumptions often unravel as it becomes apparent that interactions operate at many levels, and that firm distinctions are elusive and assurances of a complete mechanism of action may be hasty. Interestingly, many combinations described as interactions are not really interactions in the narrow technical definition. Although some may be “interactions” in some broader definition, a large proportion of adverse events derive from situations more accurately described as “contraindications” or “inappropriate prescriptions” (e.g., excessive additive effects; contrary actions; effective but inappropriately sequenced interventions; patients too young, compromised, or otherwise inappropriate for a certain agent[s] at the given dose).
Within the interactions literature, scant attention has been paid to the methodology by which interactions are analyzed. Borgert and associates (2005) noted in this connection that the “commonest approach was the no method approach.” In general, there is insufficient quantitative dose-response data in herb and nutrient interaction studies to meet rigorous criteria for the accurate demonstration of supra-additive and subadditive effects of different dose levels for a given pair of agents.
The Interactions Universe
The evaluation schema and detailed definitions and standards used in this text are provided in the following Interactions Evaluation Guide. The primary emphasis of this taxonomy is to establish an operational characterization of each interaction pair based on clinical priorities. In summary, the type and clinical significance of each known or potential interaction are parsed according to several variables: pharmacokinetic, pharmacodynamic, or clinical/strategic; adverse, beneficial, or bimodal (bidirectional); prevention or reduction of adverse effects; compensatory or protective response to probable depletions; negligible, cautionary, or avoidance levels of probable effects; and suitability for self-care or necessity of professional management. The levels of probability of clinically significant interaction are graded as “1. Certain,” “2. Probable,” “3. Possible,” “4. Plausible,” “5. Improbable,” or “6. Unknown.” In a parallel assessment, the available and reviewed evidence is evaluated according to strength and quality: “Consensus,” “Emerging,” “Preliminary,” “Mixed,” or “Inadequate.” The overall position of each interaction pair within this taxonomy is clearly data dependent, and when the available data suggested ambiguity or conflict, a final assignation was established by review and agreement of the entire editorial team.
In this text, all interactions are considered as potentially operating on several levels within the genomic variability and physiology of individual patients, their behavior and local environment, the clinical strategy and therapeutic relationship(s), and among practitioners. These multiple levels may be represented as a set of concentric circles (see figure).
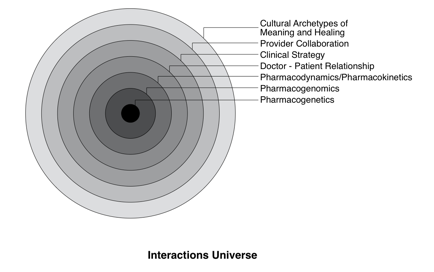
Within this “interactions universe,” multiple interventions are
more often the rule than the exception, and interactions may be influenced
by several different levels; as always, these effects may vary based on patient
characteristics and the timing relationship between different interventions.
Some interactions may be significantly affected by pharmacogenomic variability,
with those involving warfarin, statin drugs, or folic acid being prime examples.
In other cases, by shifting the perspective to clinical strategy, what may
appear superficially as an interaction may simply be a clinical contraindication;
that is, the therapeutic intent of one agent is antagonistic to the other agent,
and these would generally be avoided in combination. More subtly, concomitant
use of two agents may be characterized as “divergent” or “distracting,” in
which the action of a secondary or adjuvant therapy could theoretically reduce
the action of the primary agent and thereby reduce overall therapeutic efficacy.
A similar interaction pattern might be described as “dissonant” when
two interventions are potentially appropriate but often can be incompatible
in their action or mode of administration. Likewise, from a strategic perspective,
some agents in pairs or in clusters, particularly when applied in a logical
sequence, may act in a “consonant” manner. Thus, two agents given
simultaneously may provide minimal benefit but administered sequentially may
enhance therapeutic outcomes through strategic synergy. Two examples of such
patterns are seen in the relationship between antibiotics and probiotic flora
and, in a more complex form, between chemotherapy and antioxidants. Further,
as recognition of herbs and nutrients as therapeutic agents, rather than mere
supplements, grows, so does the need for expanding study of interactions between
and among herbs and nutrients. Classical herbal traditions all have guidelines
regarding synergies and contraindications for coadministration; such a methodology
is inherent to formula building. However, research is only coming into the
light in regard to such phenomena as the potential adverse effects on healthy
intestinal bacterial flora from herbs with direct antibacterial activity, as
opposed to indirect immune-enhancing activity. Once again, we see the need
to place all substances with known or potential pharmacological activity on
a level playing field using objective research design and integrative clinical
approaches.
The complexities of these various influences on drug activity are rarely discussed in the literature with regard to resultant “interactions” and possible clinical implications. For example, clinicians of many modalities might recommend exercise, but how often do they consider the potential impact of exercise on drug metabolism? Might not a sudden increase in physical activity significantly change clearance of certain drugs as much as or more than many common nutrients or herbs? Similarly, in an era when every patient (except those on warfarin) is advised to “eat more fruits and vegetables,” how can we calculate the impact of a significant change in dietary habits, especially for the better? Do we want to discourage patient initiative and motivation? Here, for example, one might consider the use of pomegranate juice in the patient with a family history of prostate cancer, or high intake of vegetables in someone with a family history of vascular disease. The recent finding that pomegranate juice, like grapefruit juice, inhibits the 3A4 isotype of cytochrome P450 enzymes involved in the metabolism of many pharmaceuticals, adds another level of complexity to prescribing and pharmacovigilance. Further, when the recommendation of dietary fiber and healthy oils is almost universal, doesn’t every clinician need to advise patients about the known pharmacokinetic effects on drug metabolism of increasing fiber (which can bind many medications) or of eating more fish or olive oil, with the unknown implications of the effects of lipids on simultaneously consumed drugs? In considering these possibilities, we realize the limitations of scientific knowledge and clinical experience regarding pharmacokinetics of dietary intake, which does not even take into account the wide, pharmacogenomically determined variability among patients in the activity of hepatic enzymes and other systems of detoxification.
Clinical Implications
The questions raised here and throughout this text challenge the attentive reader to reconsider drug activity within the full context of therapeutic strategy and patient outcomes. Simply put, is it a higher priority to manage therapies for the sake of the patients or for the stability of their drug levels? Ultimately, the question arises: when do we counsel patients to avoid healthy behavior on the basis of the possible risk of disrupting predictable drug levels? Even with such cautions and qualifications, can we be certain this is really obtainable? How does such an approach address interindividual biochemical and metabolic variability? These issues have been discussed in nutrition therapeutics for decades (e.g., Roger Williams, PhD, discoverer of pantothenic acid and father of the concept of “metabolic individuality”) and are foundational to the methodology of many of the so-called alternative medical traditions. For example, after emphasizing to patients the importance of the modern mantra of “eat right and exercise,” how do we accept the paradox of telling “warfarinized” patients with cardiovascular disease to refrain from eating green vegetables, while also asserting repeatedly that food sources of nutrients are inherently superior to pharmaceutical supplemental sources?
Most experienced health care professionals understand that many “disruptive” behaviors (in terms of medication levels) are not really “problematic” in an absolute sense, and that simply refraining from such behaviors is not the answer. In fact, the patient who abruptly stops using a nutrient or herb, drops something from the diet, or radically changes habits out of fear and ill-informed warnings is actually at greater risk from sudden changes. Carried to its extreme, might we not recommend to patients that they sit on the couch and eat only nonnutritive food so as best to maintain stable drug levels? Do we want our patients to imitate the in vitro experiments that we so often caution ourselves not to extrapolate so freely into human physiology? Thus, for example, with patients receiving oral anticoagulants, it would be more desirable, both from a health perspective and in terms of building patient rapport, if we could work with them on increasing healthy foods in their diet, teaching them to maintain a stable intake of dietary vitamin K, while monitoring their INR and titrating it with the appropriate warfarin dosage to maintain the desired clinical effect.
In reviewing the broad literature relevant to interactions between drugs and natural products, four key factors seem paramount and decisive to safety and efficacy: doctor-patient communication and trust; therapeutic index and rapidity of response; monitoring, feedback, and titration; and the urgent need for high-quality research and well-documented case reports together with communication and collaboration. Moreover, the overview of all the findings involving such interactions strongly suggests that other than a minority of clearly dangerous and contraindicated combinations of agents, most interactions are therapeutically advantageous when managed properly, and some portion are between these two polarities, where they pose clinically significant risks only when frank discussion and full disclosure are lacking and corrective measures are not implemented. In some cases, nutritional and herbal therapies may impair activity of a drug by promoting healthy physiological functions (our primary goal) and thereby increase metabolism of stressful or toxic substances, including many drugs. Thus, in these circumstances, the issue is not necessarily incompatibility, but rather lack of coordination and inappropriate timing, such as separation of bile sequestrant intake from the ingestion of fat-soluble nutrients, which tend to be depleted by such a drug, or separating intake of an immune-supportive nutrient such as zinc from antibiotics when the two might chelate when ingested simultaneously.
Although some observations and recommendations in this text may appear unfamiliar to some medical practitioners, the material used is inherently conservative, and the clinical suggestions are designed to be both pragmatic and clinically oriented, with an emphasis on objectivity and evidence, safety and efficacy. Apart from research into certain well-known drug-induced nutrient depletion patterns, direct clinical trials of adequate power are nearly absent from literature pertaining to drug-herb and drug-nutrient interactions. The scant number of reports of well-qualified and clinically significant adverse events suggests that dangers may be less than some have anticipated or declared, especially given the widespread use of herbs and nutrients and the overlap with medication intake. Moreover, research directed at the potential value of the anecdotal evidence available in qualified case reports strongly suggests that these could be a resource of premier value, given the complexities of interactions and the metabolic idiosyncrasies of patients.
Drug-induced nutrient depletion patterns constitute a significant proportion of drug-nutrient interactions. In these situations, awareness of interference with physiological functions of key nutrients or simple decline in available nutrient levels contributes directly or indirectly to known adverse drug effects and offers simple and safe interventions for reducing adverse effects, increasing patient comfort and compliance, and improving therapeutic efficacy and clinical outcomes. Research in this area was largely established in professional discourse by Daphne A. Roe, MD, who focused on such phenomena, particularly in geriatric patients, more than three decades ago. Growing awareness of nutrient use by patients and interest in nutritional therapies within conventional medicine suggest that this body of scientific literature will expand in coming years.
In essence, the issue of interactions presents physicians and other health care professionals with the challenge of working as allies, treating all interventions as options to be evaluated as tactics with a potential to enhance the therapeutic strategy. Thus, we may come to reframe the term “alternative medicine” not as “unconventional” or “competing” but rather as a range of options within a comprehensive repertoire to be considered in light of the patients’ risks, needs, and history, as well as their preferences and values, motivation and compliance. Furthermore, effective clinical practice requires more flexible approaches, given the limitations of predictive models, especially when we question whether any model can accurately comprehend and predict the outcomes if more than two factors interact in a patient whose natal pharmacogenomic individuality has been modified by a lifetime's layers of stressors, trauma, and supports. These complexities suggest the need for an evolving and customized response in providing medical care, especially when treating chronic disease, in which some generic elements of treatment intertwine with some highly individualized aspects, and all components shift through phases of the clinical strategy and are crafted to optimize multiple interventions. The nature of the therapeutic relationship and its central role in such a setting emphasize the primacy of establishing and maintaining trust and frank open communication, the ability to be supportive and challenging, especially regarding diet and other lifestyle factors, and the need to be nonjudgmental, flexible, and responsive.
These issues point to patient self-care and utilization of multiple health care professionals from different modalities or specializations as central but often-ignored factors in the clinical reality of drug-herb and drug-nutrient interactions. In discussions of diverse approaches to health care and medicine, two distinct but often-related issues often become confused and inappropriately intermingled.
First, patients are using a wide range of medical treatments, ingesting unsupervised and untested permutations of substance combinations, and experimenting with both ancient and novel techniques and behaviors for enhancing wellness and treating disease. Since the early 1990s, conventional medicine has become increasingly aware of the patterns of utilization of health care services provided by health care professionals other than medical physicians (MDs), as well as use of nonconventional therapies by MDs. Likewise, the emerging educational, legal, and professional infrastructures of accredited educational institutions, professional associations, and licensing laws reveal the continued growth of naturopathic physicians, chiropractors, acupuncturists, massage therapists, medical herbalists, and others. Providing safe and effective clinical care in this environment requires knowledge, mutual respect, communication, and collaboration among health care providers working with individual patients, recognizing, affirming, and utilizing their choices, instincts, experiences, and intuitions. These issues become even more complex when patients have ready access to previously unavailable medical information and demand a partnership based on informed decision making. Professionals may have difficulty accepting that each patient, especially patients with chronic disease, holds this final responsibility and control and that health care providers are simply participants in this critical aspect of the patient's life and personal evolution.
Demographic data indicate that higher educational and income levels are associated with the use of natural medicine and alternative therapies. Whether acting from healthy initiative or desperation, these patients deserve respect in their health care choices and support in obtaining coherent medical care. Self-care is a strong tradition within American, English, and other cultures. Whether Culpepper or the Thomsonian and Hygienic movements, individuals, families, and communities have long fought for their right to continue their traditions of care that predate professional medicine. Although potentially challenging to our authority as educated health care professionals (and to the time constraints of daily practice), education and initiative, informed decision making, and self-empowerment can be our greatest allies in promoting health, preventing illness, and treating disease, with proper timing and strategic coordination. Here, must we not defer to the obvious necessity and professional duty to build trust based on honesty and full disclosure? Enabling frank discussions can provide the most reliable means of respecting patients’ choices and satisfying their needs within a context of communication and mutual respect, collaboration and coordination among an integrative team of health care professionals.
This brings us to the second broad and often-unstated issue deeply involved in adverse events and interactions involving patients utilizing the services of multiple health care providers. Short of learning many types of medicine directly, clinicians of every type, whether conventional MDs, practitioners of natural medicine traditions, or practitioners from indigenous medical traditions, can benefit from cultivating collegial alliances with professionals from the diverse health care traditions that their patients utilize, as a means of developing practical familiarity and experience in cross-referrals. Each of these schools of medicine possesses its own respective models, techniques, and tools, and each derives from unique circumstances of history, demographics, and culture. Although simply the result of a lack of opportunity in some cases, ignorance of other health care systems often results from indefensible reliance on ill-founded rumors and inherited prejudices. Rarely does an MD really know the particulars of the chiropractic profession, or vice versa. Likewise, many practitioners of nutritional therapies have little substantive training in herbal medicine, and vice versa, to say nothing of the huge differences among European phytotherapy, American botanical medicine, and Chinese herbal tradition. Unfortunately, besides the notable exception of numerous pharmacists, our patients sometimes have a broader view of the numerous medical options in their repertoire than their health care providers, and that knowledge base is often far wider than deep. None of these parochial attitudes and behaviors serves the interest of our patients or respects their choices and values. Our responsibility as health care professionals is to serve our patients’ needs, have access to information regarding the substances they choose to ingest and the procedures they find beneficial, and build collaborative relationships with providers experienced in those approaches, regardless of whether we agree with or support those choices. Ultimately, is any other option professionally ethical, clinically responsible, or therapeutically effective?
Reports of adverse events or interactions involving nutritional therapies or botanical agents almost universally derive from situations of patient self-medication, usually without disclosure or coordinated planning by the health care providers, and often involve faulty preparation, adulteration, contamination, or other departures from typical responses to known interventions. In contrast, substantive reports of adverse events or interactions regarding nutritional therapies or botanical agents prescribed, administered, and supervised by health care professionals trained and experienced in the respective therapies are virtually nonexistent. The few exceptions typically represent interventions considered within the standards of care but subsequently determined to be inappropriate to specific patient populations or in particular dosages or preparations. Thus, MDs, naturopathic physicians, and qualified herbalists have rarely been involved in situations of adverse reactions or interactions with prescribed nutritional or herbal treatments; in fact, they usually are enthusiastic advocates of scientific research through well-designed trials that might ensure safety, clarify indications, and enhance efficacy.
Based on our education, training, and clinical experience and strongly influenced by the process of compiling and analyzing the material in this text, we offer the following final thoughts. First, the issue of interactions involves not only avoiding unnecessary risks, but also recognizing unforeseen opportunities. The greater promise in integrative medicine lies not in the use of naturally occurring agents, such as an herb, food, or nutrient, to treat standard diagnoses in place of pharmaceutical agents, nor in the benefits of expanding the clinical repertoire of conventional medicine, but in rendering its medicines more effective, especially in combination with innovations in pharmacogenomics, systems physiology, and new research methodologies. More profoundly, the very movement of engaging with transdisciplinary approaches and their underlying models offers all practitioners and providers, conventional or otherwise, an inseparable corollary to the restructuring of their practice, the potential for transformation and expansion of their own consciousness, and an openness to a greater vision of the mysterious and miraculous in medicine.
Mitchell Bebel Stargrove, ND, Lac
Jonathan Treasure MA, MNIMH, RH(AHG)
Dwight L. McKee, MD
Bibliography
- Adams KM, Lindell KC, Kohlmeier M and Zeisel SH: Status of nutrition education in medical schools. Am J Clin Nutr. 83(4):2006; 941S–944S.
- Aiken LH: Achieving an interdisciplinary workforce in health care. N Engl J Med. 348:2003; 164–166.
- Anonymous. Complementary and alternative medicine: what people 50 and over are using and discussing with their physicians. National Center for Complementary and Alternative Medicine. Accessed January 2007. http://nccam.nih.gov/timetotalk/.
- Armstrong SC, Cozza KL and Sandson NB: Six patterns of drug-drug interactions. Psychosomatics. 44(3):2003; 255–258.
- Aronson JK: Unity from diversity: the evidential use of anecdotal reports of adverse drug reactions and interactions. J Eval Clin Pract. 11(2):2005; 195–208.
- Aronson JK, Hauben M. Anecdotes that provide definitive evidence. BMJ, 2006;16;333(7581):1267-1269 (review).
- Astin JA, Pelletier KR, Marie A and Haskell WL: Complementary and alternative medicine use among elderly persons: one-year analysis of a Blue Shield Medicare supplement. J Gerontol A Biol Sci Med Sci. 55(1):2000; M4–M9.
- Berman B, Bausell R and Lee W-L: Use and referral patterns for 22 complementary and alternative medical therapies by members of the American College of Rheumatology: results of a national survey. Arch Intern Med. 162(7):2002; 766–770.
- Borgert CJ, Borgert SA and Findley KC: Synergism, antagonism, or additivity of dietary supplements: application of theory to case studies. Thromb Res. 117(1-2):2005; 123–132.
- Casagrande SS, Wang Y, Anderson C and Gary TL: Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am J Prev Med. 32(4):2007; 257–263.
- Cherkin DC, Deyo RA and Sherman KJ, et al.: Characteristics of visits to licensed acupuncturists, chiropractors, massage therapists, and naturopathic physicians. J Am Board Fam Pract. 15(6):2002; 463–472.
- Davis DR, Epp MD and Riordan HD: Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J Am Coll Nutr. 23:2004; 669–682.
- Druss BG, Marcus SC and Olfson M, et al.: Trends in care by nonphysician clinicians in the United States. N Engl J Med. 348(2):2003; 130–137.
- Druss BG and Rosenheck RA: Association between use of unconventional therapies and conventional medical services. JAMA. 282(7):1999; 651–656.
- Eisenberg DM, Kessler RC and Foster C, et al.: Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med. 328(4):1993; 246–252.
- Eisenberg DM, Kessler RC and Van Rompay MI, et al.: Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 135(5):2001; 344–351.
- Fugh-Berman A and Ernst E: Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol. 52(5):2001; 587–595.
- Ginsburg GS, Konstance RP, Allsbrook JS and Schulman KA: Implications of pharmacogenomics for drug development and clinical practice. Arch Intern Med. 165(20):2005; 2331–2336., (review)
- Goldstein M, Brown ER and Ballard-Barbash R, et al.: The use of complementary and alternative medicine among California adults with and without cancer. eCAM. 2:2005; 557–565.
- Grinnell F, Bishop JP and McCullough LB: Bioethical pluralism and complementarity. Perspect Biol Med. 45(3):2002; 338–349., (review)
- Harnack LJ, Rydell SA and Stang J: Prevalence of use of herbal products by adults in the Minneapolis/St Paul, Minn, metropolitan area. Mayo Clin Proc. 76(7):2001; 688–694.
- Hidaka M, Okumura M and Fujita K, et al.: Effects of pomegranate juice on human cytochrome P450 3A (CYP3A) and carbamazepine pharmacokinetics in rats. Drug Metab Dispos. 33(5):2005; 644–648.
- Howard N, Tsourounis C and Kapusnik-Uner J: Dietary supplement survey of pharmacists: personal and professional practices. J Altern Complement Med. 7(6):2001; 667–680.
- Jadad AR, Moore RA and Carroll D, et al.: Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials. 17(1):1996; 1–12.
- Kaptchuk TJ: The double-blind, randomized, placebo-controlled trial: gold standard or golden calf?. J Clin Epidemiol. 54(6):2001; 541–549.
- Kaptchuk TJ and Eisenberg DM: Varieties of healing. 1. Medical pluralism in the United States. Ann Intern Med. 135:2001; 189–195.
- Kessler RC, Davis RB and Foster DF, et al.: Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 135(4):2001; 262–268.
- Lafferty WE, Bellas A and Corage Baden A, et al.: The use of complementary and alternative medical providers by insured cancer patients in Washington State. Cancer. 100(7):2004; 1522–1530.
- Laine C, Goodman SN, Griswold ME and Sox HC: Reproducible research: moving toward research the public can really trust. Ann Intern Med. 146(6):2007; 450–453.
- Leung JM, Dzankic S, Manku K and Yuan S: The prevalence and predictors of the use of alternative medicine in presurgical patients in five California hospitals. Anesth Analg. 93(4):2001; 1062–1068.
- Mann HJ: Drug-associated disease: cytochrome P450 interactions. Crit Care Clin. 22(2):2006; 329–345., vii (review)
- Millen AE, Dodd KW and Subar AF: Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: the 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc. 104(6):2004; 942–950.
- Pal D and Mitra AK: MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 78(18):2006; 2131–2145., (review)
- Paramore LC: Use of alternative therapies: estimates from the 1994 Robert Wood Johnson Foundation National Access to Care Survey. J Pain Symptom Manage. 13(2):1997; 83–89.
- Pelletier KR, Marie A, Krasner M and Haskell WL: Current trends in the integration and reimbursement of complementary and alternative medicine by managed care, insurance carriers, and hospital providers. Am J Health Promot. 12(2):1997; 112–122., (review)
- Roe D, Drug-Induced Nutritional Deficiencies. 1976; Westport, Conn: AVI Publishing
- Rothwell PM: External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 365(9453):2005; 82–93.
- Rothwell PM: Treating individuals. 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 365(9454):2005; 176–186.
- Rothwell PM, Mehta Z and Howard SC, et al.: Treating individuals. 3. From subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet. 365(9455):2005; 256–265.
- Stamford BA. Curing health and medical coverage. Am Journalism Rev 2000;56-59.
- Tang C, Lin JH and Lu AY: Metabolism-based drug-drug interactions: what determines individual variability in cytochrome P450 induction?. Drug Metab Dispos. 33(5):2005; 603–613., (review)
- Treasure J. MEDLINE and the mainstream manufacture of misinformation. Herbal Hypotheses 2;2006. http://www.herbological.com/downloads.html.
- Treasure J. Warding off evil in the 21st century: St. John's wort as a xenosensory activator. Herbal Hypotheses 1;2005. http://www.herbological.com/downloads.html.
- Votova K and Wister AV: Self-care dimensions of complementary and alternative medicine use among older adults. Gerontology. 53(1):2007; 21–27.
You, the reader, are invited to send additions, corrections, citations, and other input and feedback to Interactions2@MedicineWorks.com
